Text Practice Mode
Building the Periodic Table of the Elements Dmitri Mendeleev, a Russian chemist and teacher, devised the periodic table — a comprehensive system for classifying the chemical elements. Dmitri Mendeleev in 1897, public domain Organizing Matter In the mid-17
created Mar 12th 2022, 05:41 by Ajit kumar Pani
1
981 words
8 completed
2.5
Rating visible after 3 or more votes
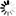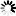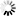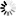 saving score / loading statistics ...
saving score / loading statistics ...
00:00
Building the Periodic Table of the Elements
Dmitri Mendeleev, a Russian chemist and teacher, devised the periodic table — a comprehensive system for classifying the chemical elements.
Dmitri Mendeleev in 1897, public domain
Organizing Matter
In the mid-1700s, chemists began actively identifying elements, which are substances made up of just one kind of atom. But a century later, they still used a variety of symbols and acronyms to represent the different materials — there just wasn’t a common lexicon. In 1869, the Russian chemist Dmitri Mendeleev came to prominence with his tabular diagram of known elements. This basic ingredient list, of which all matter exists, became known as the periodic table. Here’s what’s especially amazing: Mendeleev’s chart allotted spaces for elements that were yet to be discovered. For some of these missing pieces, he predicted what their atomic masses and other chemical properties would be. When scientists later discovered the elements Mendeleev expected, the world got a glimpse of the brilliance behind the periodic table.
An engraving of Mendeleev, public domain
A Difficult Childhood
Mendeleev was born in 1834 in the far west of Russia’s Siberia, the youngest of a dozen or more children (reports vary). His family faced one crisis after another. When Dmitri was little, his father, a teacher, went blind, and his mother went to work. She became the manager of a successful glass factory. Tragedy struck again in 1848, when the factory burned down, and the family faced poverty. Mendeleev’s mother was determined to get him an education, and traveled with him a great distance, to Moscow and then to St. Petersburg, to do so. Ten days after he was enrolled in school, his mother died of tuberculosis, a disease that had also taken his father, at least one of his siblings, and that Mendeleev himself would battle as a young adult.
A Young Professor
In 1861, Mendeleev returned to Russia from research in Europe and later taught at the Technical Institute in St. Petersburg. He found that few of the new developments in the field of chemistry had made their way to his homeland — something he was determined to change, lecturing enthusiastically about the latest advances. Only 27 years old, he cultivated the persona of an eccentric, with a flowing beard and long, wild hair that he was known to trim only once a year. Still, he was a popular professor.
Mendeleev recognized that there was no contemporary textbook on modern organic chemistry (concerned with carbon compounds, including living things), so he wrote one. His Organic Chemistry (1861) was considered his era’s most authoritative book on the subject. But the professor was painfully aware that many of his students “could not follow” him, as one student observed. Mendeleev knew that a critical reason for peoples’ difficulty in understanding chemistry was the lack of any clear system for classifying the known elements. Without one, he could only offer particulars about specific building blocks of matter, but no framework that would explain the relationships between different substances.
As Mendeleev wrote:
The edifice of science requires not only material, but also a plan, and necessitates the work of preparing the materials, putting them together, working out the plans and symmetrical proportions of the various parts. (Strathern, 2000)
A Missed Train and a Dream
Next, Mendeleev began a text for inorganic chemistry (concerned with substances that are not organic, such as minerals), and the result, Principles__of Chemistry (two volumes, 1868–1870), would become the standard text for the field until early in the 20th century. His research for this book would also lead him to his most renowned work.
In 1867, when Mendeleev began writing Principles of Chemistry, he set out to organize and explain the elements. He began with what he called the “typical” elements: hydrogen, oxygen, nitrogen, and carbon. Those substances demonstrated a natural order for themselves. Next he included the halogens, which had low atomic weights, reacted easily with other elements, and were readily available in nature. He had begun by using atomic weights as a principle of organization, but these alone did not present a clear system.
At the time, elements were normally grouped in two ways: either by their atomic weight or by their common properties, such as whether they were metals or gases. Mendeleev’s breakthrough was to see that the two could be combined in a single framework.
Mendeleev was said to have been inspired by the card game known as solitaire in North America, and “patience” elsewhere. In the game, cards are arranged both by suit, horizontally, and by number, vertically. To put some order into his study of chemical elements, Mendeleev made up a set of cards, one for each of the 63 elements known at the time. Mendeleev wrote the atomic weight and the properties of each element on a card.
He took the cards everywhere he went. On February 17, 1869, right after breakfast, and with a train to catch later that morning, Mendeleev set to work organizing the elements with his cards. He carried on for three days and nights, forgetting the train and continually arranging and rearranging the cards in various sequences until he noticed some gaps in the order of atomic mass.
As one story has it, Mendeleev, exhausted from his three-day effort, fell asleep. He later recalled, “I saw in a dream, a table, where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.” (Strathern, 2000) He named his discovery the “periodic table of the elements.”
After his dream, Mendeleev drew the table he had envisioned. While arranging these cards of atomic data, Mendeleev discovered what is called the Periodic Law. When Mendeleev arranged the elements in order of increasing atomic mass, the properties where repeated. Because the properties repeated themselves regularly, or periodically, on his chart, the system became known as the periodic table.
Dmitri Mendeleev, a Russian chemist and teacher, devised the periodic table — a comprehensive system for classifying the chemical elements.
Dmitri Mendeleev in 1897, public domain
Organizing Matter
In the mid-1700s, chemists began actively identifying elements, which are substances made up of just one kind of atom. But a century later, they still used a variety of symbols and acronyms to represent the different materials — there just wasn’t a common lexicon. In 1869, the Russian chemist Dmitri Mendeleev came to prominence with his tabular diagram of known elements. This basic ingredient list, of which all matter exists, became known as the periodic table. Here’s what’s especially amazing: Mendeleev’s chart allotted spaces for elements that were yet to be discovered. For some of these missing pieces, he predicted what their atomic masses and other chemical properties would be. When scientists later discovered the elements Mendeleev expected, the world got a glimpse of the brilliance behind the periodic table.
An engraving of Mendeleev, public domain
A Difficult Childhood
Mendeleev was born in 1834 in the far west of Russia’s Siberia, the youngest of a dozen or more children (reports vary). His family faced one crisis after another. When Dmitri was little, his father, a teacher, went blind, and his mother went to work. She became the manager of a successful glass factory. Tragedy struck again in 1848, when the factory burned down, and the family faced poverty. Mendeleev’s mother was determined to get him an education, and traveled with him a great distance, to Moscow and then to St. Petersburg, to do so. Ten days after he was enrolled in school, his mother died of tuberculosis, a disease that had also taken his father, at least one of his siblings, and that Mendeleev himself would battle as a young adult.
A Young Professor
In 1861, Mendeleev returned to Russia from research in Europe and later taught at the Technical Institute in St. Petersburg. He found that few of the new developments in the field of chemistry had made their way to his homeland — something he was determined to change, lecturing enthusiastically about the latest advances. Only 27 years old, he cultivated the persona of an eccentric, with a flowing beard and long, wild hair that he was known to trim only once a year. Still, he was a popular professor.
Mendeleev recognized that there was no contemporary textbook on modern organic chemistry (concerned with carbon compounds, including living things), so he wrote one. His Organic Chemistry (1861) was considered his era’s most authoritative book on the subject. But the professor was painfully aware that many of his students “could not follow” him, as one student observed. Mendeleev knew that a critical reason for peoples’ difficulty in understanding chemistry was the lack of any clear system for classifying the known elements. Without one, he could only offer particulars about specific building blocks of matter, but no framework that would explain the relationships between different substances.
As Mendeleev wrote:
The edifice of science requires not only material, but also a plan, and necessitates the work of preparing the materials, putting them together, working out the plans and symmetrical proportions of the various parts. (Strathern, 2000)
A Missed Train and a Dream
Next, Mendeleev began a text for inorganic chemistry (concerned with substances that are not organic, such as minerals), and the result, Principles__of Chemistry (two volumes, 1868–1870), would become the standard text for the field until early in the 20th century. His research for this book would also lead him to his most renowned work.
In 1867, when Mendeleev began writing Principles of Chemistry, he set out to organize and explain the elements. He began with what he called the “typical” elements: hydrogen, oxygen, nitrogen, and carbon. Those substances demonstrated a natural order for themselves. Next he included the halogens, which had low atomic weights, reacted easily with other elements, and were readily available in nature. He had begun by using atomic weights as a principle of organization, but these alone did not present a clear system.
At the time, elements were normally grouped in two ways: either by their atomic weight or by their common properties, such as whether they were metals or gases. Mendeleev’s breakthrough was to see that the two could be combined in a single framework.
Mendeleev was said to have been inspired by the card game known as solitaire in North America, and “patience” elsewhere. In the game, cards are arranged both by suit, horizontally, and by number, vertically. To put some order into his study of chemical elements, Mendeleev made up a set of cards, one for each of the 63 elements known at the time. Mendeleev wrote the atomic weight and the properties of each element on a card.
He took the cards everywhere he went. On February 17, 1869, right after breakfast, and with a train to catch later that morning, Mendeleev set to work organizing the elements with his cards. He carried on for three days and nights, forgetting the train and continually arranging and rearranging the cards in various sequences until he noticed some gaps in the order of atomic mass.
As one story has it, Mendeleev, exhausted from his three-day effort, fell asleep. He later recalled, “I saw in a dream, a table, where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.” (Strathern, 2000) He named his discovery the “periodic table of the elements.”
After his dream, Mendeleev drew the table he had envisioned. While arranging these cards of atomic data, Mendeleev discovered what is called the Periodic Law. When Mendeleev arranged the elements in order of increasing atomic mass, the properties where repeated. Because the properties repeated themselves regularly, or periodically, on his chart, the system became known as the periodic table.
 saving score / loading statistics ...
saving score / loading statistics ...